 The Optical Coherence Microscope (OCM) at Harvey Mudd College is funded primarily
by a National Science Foundation grant (DBI-9612240) to construct an instrument
useful in the study of fundamental problems in developmental biology. Accordingly,
we have developed the OCM to image critical events
in the early development of plants and animals that are inaccessible using
conventional microscopy techniques.
Optical coherence microscopy is capable of non-invasively, non-destructively imaging cells or groups of cells
located up to one millimeter below the surface of living tissue, regions rendered opaque
using two-photon or confocal microscopy. Because are capable of imaging structures this far below the
tissue surface, we have designed our OCM to acquire three-dimensional
data sets of biological tissue. Thus, we are able to acquire three-dimensional
time-lapse movies of tissue development in vivo, that is, without disturbing the
regular growth and changes occuring within the sample.
The Optical Coherence Microscope (OCM) at Harvey Mudd College is funded primarily
by a National Science Foundation grant (DBI-9612240) to construct an instrument
useful in the study of fundamental problems in developmental biology. Accordingly,
we have developed the OCM to image critical events
in the early development of plants and animals that are inaccessible using
conventional microscopy techniques.
Optical coherence microscopy is capable of non-invasively, non-destructively imaging cells or groups of cells
located up to one millimeter below the surface of living tissue, regions rendered opaque
using two-photon or confocal microscopy. Because are capable of imaging structures this far below the
tissue surface, we have designed our OCM to acquire three-dimensional
data sets of biological tissue. Thus, we are able to acquire three-dimensional
time-lapse movies of tissue development in vivo, that is, without disturbing the
regular growth and changes occuring within the sample.
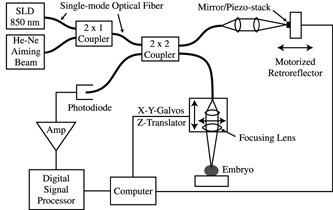 Optical Coherence Microscopy is based on Michelson Interferometry, an experimental
technique which measures the interference fringes caused by light from a single source traveling
the same distance along two different optical paths. The OCM gathers information from the inside
of the sample by focusing the beam spot (waist) at an equal path length position
that is below the surface tissue layer and recording the intensity of directly back-scattered
light. This is essentially a measure of the cross-sectional scattering area within a particular
volume element. Multiply-scattered light from out-of-focus planes within the sample is filtered out
by the coherence gate of our Michelson Interferometer setup, preserving high spatial resolution
even within the sample. The OCM Schematic on
this page illustrates the setup of the instrument, including the
two separate optical paths, one consisting of a reference mirror, the other of the sample to be imaged.
The light source our OCM uses is a super-luminescent diode (SLD) operating at 843 nm with a spectral width
of 21 nm full-width-half-maximum (FWHM). This setup achieves depth resolution of 15 micrometers
in air or approximately 10 micrometers in tissue (n~1.4), which is determined by the coherence length of the SLD.
The lateral resolution of our instrument corresponds to the waist of the focused beam in the
sample arm, about 5 micrometers FWHM. This spatial resolution defines a
volume element (voxel) where the focused beam waist is coincident with the equal path length
position of our interferometer.
Optical Coherence Microscopy is based on Michelson Interferometry, an experimental
technique which measures the interference fringes caused by light from a single source traveling
the same distance along two different optical paths. The OCM gathers information from the inside
of the sample by focusing the beam spot (waist) at an equal path length position
that is below the surface tissue layer and recording the intensity of directly back-scattered
light. This is essentially a measure of the cross-sectional scattering area within a particular
volume element. Multiply-scattered light from out-of-focus planes within the sample is filtered out
by the coherence gate of our Michelson Interferometer setup, preserving high spatial resolution
even within the sample. The OCM Schematic on
this page illustrates the setup of the instrument, including the
two separate optical paths, one consisting of a reference mirror, the other of the sample to be imaged.
The light source our OCM uses is a super-luminescent diode (SLD) operating at 843 nm with a spectral width
of 21 nm full-width-half-maximum (FWHM). This setup achieves depth resolution of 15 micrometers
in air or approximately 10 micrometers in tissue (n~1.4), which is determined by the coherence length of the SLD.
The lateral resolution of our instrument corresponds to the waist of the focused beam in the
sample arm, about 5 micrometers FWHM. This spatial resolution defines a
volume element (voxel) where the focused beam waist is coincident with the equal path length
position of our interferometer.
 Light reflected from voxels in the sample arm, as well as from the reference mirror, travels through optical fiber
to a photodetector, where it can be measured in order to determine properties of the
sample. To make this measurement, we must generate interference fringes between these two light
waves, as it is the amplitude of these fringes that can be used to determine how highly
scattering a particular voxel in the sample is. Generating fringes can be done by rapidly
oscillating the reference mirror and thus the phase difference between the two light beams.
Phase modulation in our OCM is designed specifically for fast data acquisition without
degradation of image resolution. Our approach consists of a small, lightweight reference mirror
mounted onto a piezo stack. We modulate the reference mirror through small amplitude (350-400 nm),
periodic oscillations by driving the mirror/piezo system at a resonance frequency of about 125 kHz.
This high frequency phase modulation facilitates
rapid data acquisition in our OCM. Further, since the small amplitude oscillations in the
reference path length are much smaller than our depth
resolution the blurring in the depth dimension is negligible.
More information on output fringe generation and the mirror/piezo system is available in the
Optics Express article on the
publications web page.
Light reflected from voxels in the sample arm, as well as from the reference mirror, travels through optical fiber
to a photodetector, where it can be measured in order to determine properties of the
sample. To make this measurement, we must generate interference fringes between these two light
waves, as it is the amplitude of these fringes that can be used to determine how highly
scattering a particular voxel in the sample is. Generating fringes can be done by rapidly
oscillating the reference mirror and thus the phase difference between the two light beams.
Phase modulation in our OCM is designed specifically for fast data acquisition without
degradation of image resolution. Our approach consists of a small, lightweight reference mirror
mounted onto a piezo stack. We modulate the reference mirror through small amplitude (350-400 nm),
periodic oscillations by driving the mirror/piezo system at a resonance frequency of about 125 kHz.
This high frequency phase modulation facilitates
rapid data acquisition in our OCM. Further, since the small amplitude oscillations in the
reference path length are much smaller than our depth
resolution the blurring in the depth dimension is negligible.
More information on output fringe generation and the mirror/piezo system is available in the
Optics Express article on the
publications web page.
We perform two-dimensional en face scans (in a horizontal plane normal to the incident beam) using a pair of galvoscanning mirrors to translate the waist of the focused beam. The depth of each scan plane is incremented by moving the focusing lens of the sample arm to assure a constant lateral resolution while simultaneously adjusting the position of the reference arm mirror to keep the equal path length position of the OCM interferometer coincident with the focused beam waist. This procedure maintains both the lateral and depth resolution during image acquistion. By stacking successive en face scans of increasing depths we achieve a 3-D data set which can then be processed by visualization software to produce rotatable 3-D images viewed on a computer screen. This technique of visualizing OCM data is powerful and illustrated well in the time-lapse movies and rotated images available on the plant and frog pages.
In improving the OCM instrument we are most concerned with better spatial resolution, quicker data acquisition, and increasing depth penetration. A significant improvement in spatial resolution and depth penetration has been proposed by replacing our SLD with a chromium:forsterite laser. The advantage of this light source is two-fold: First, the laser operates at 1300 nm with an output power of at least 10 mW, approximately ten times our current power. Secondly, the spectral bandwidth of the laser is about 150 nm which would yield a depth resolution of 5 micrometers in air, a sizable improvement in our spatial resolution. The combined effect of these two advantages is an increase in depth penetration by about 50%. We are also considering two major additions in data analysis that would significantly increase the information content of the OCM data. First, we would like to incorporate a Doppler mode of operation that will enable the detection of directed and brownian motion. This will help to distinguish diffusing or scattering cells from those cell groups exhibiting purposive directed motion. Second, we will implement rapid fine adjustment of the position of the focused beam waist in the sample to dynamically account for variations in refractive index in the sample. Significant increases in information and image quality would result, especially in developing organisms in which large variations of refractive index occur. Further details of OCM instrument improvement are numerous and can be found in the second NSF Proposal, also available on the publications web page.